Ronaldo V. Lobato
www.rvlobato.com
Department of Physics and Astronomy,
Texas A&M University - Commerce, Texas, USA.
Some history and basic concepts
History
1896 - Henri Becquerel discovers the radioactivity.
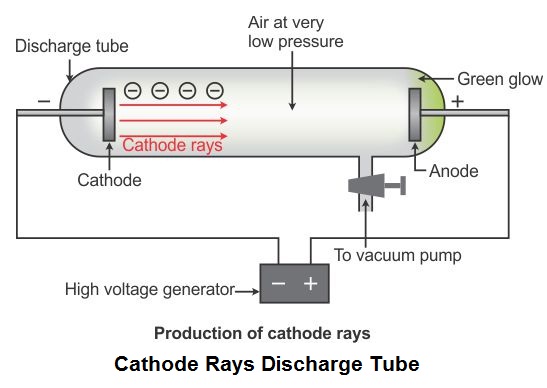
1897 - J.J. Thomson discovers the electron, indicating that the atom has internal structure.

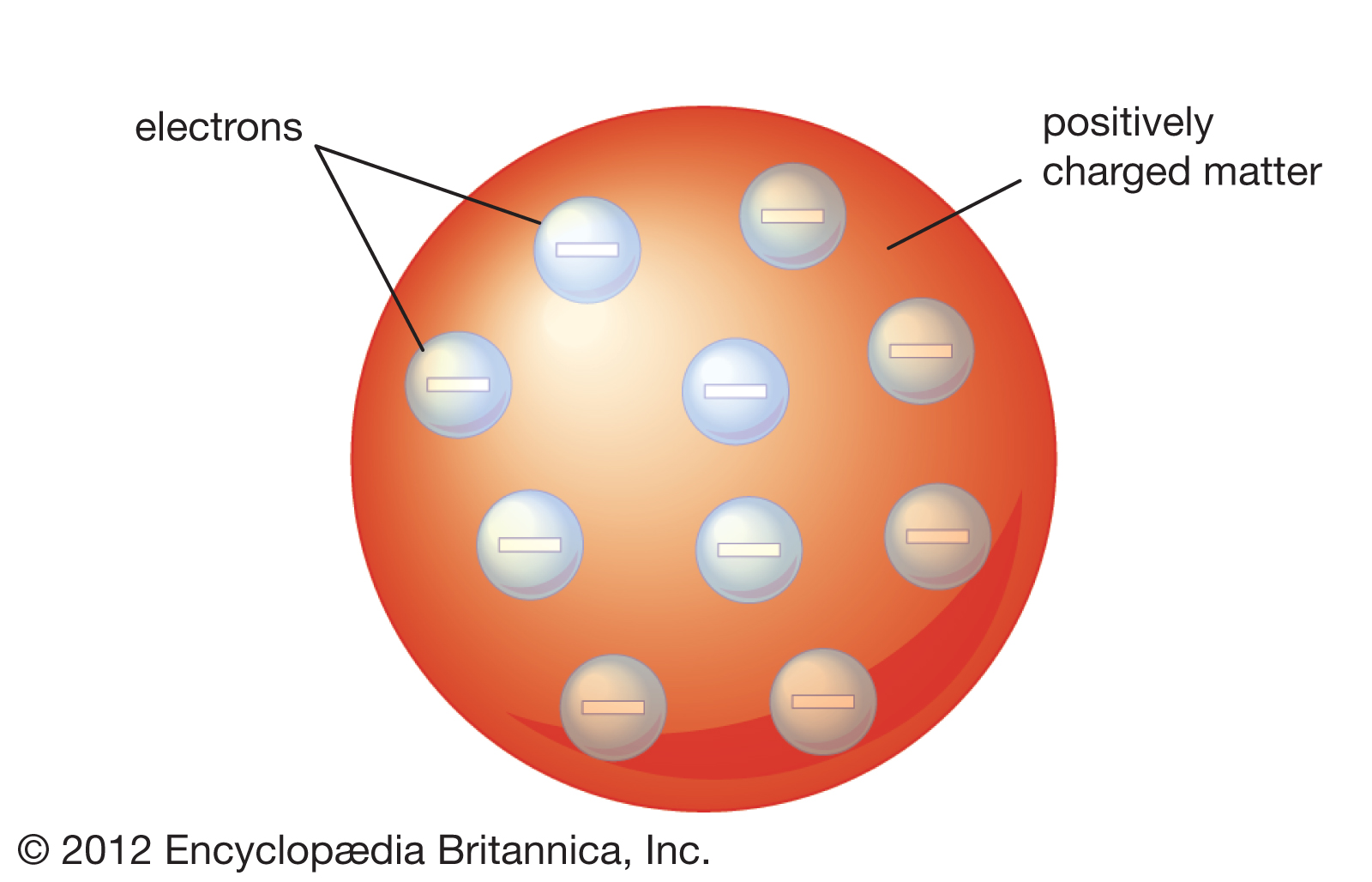
1900 - William Thomson (Lord Kelvin) proposes the first theoretical description of the atom.
Radioactivity was extensively studied by Marie, Pierre Curie, Ernest Rutherford and others



Physicists discovered three types of radiation.
1903 - Nobel prize to Becquerel and to Marie and Pierre. Rutherford won the 1908 Nobel Prize in Chemistry.
1903 - Einstein formulated the ideia of mass-energy equivalence.
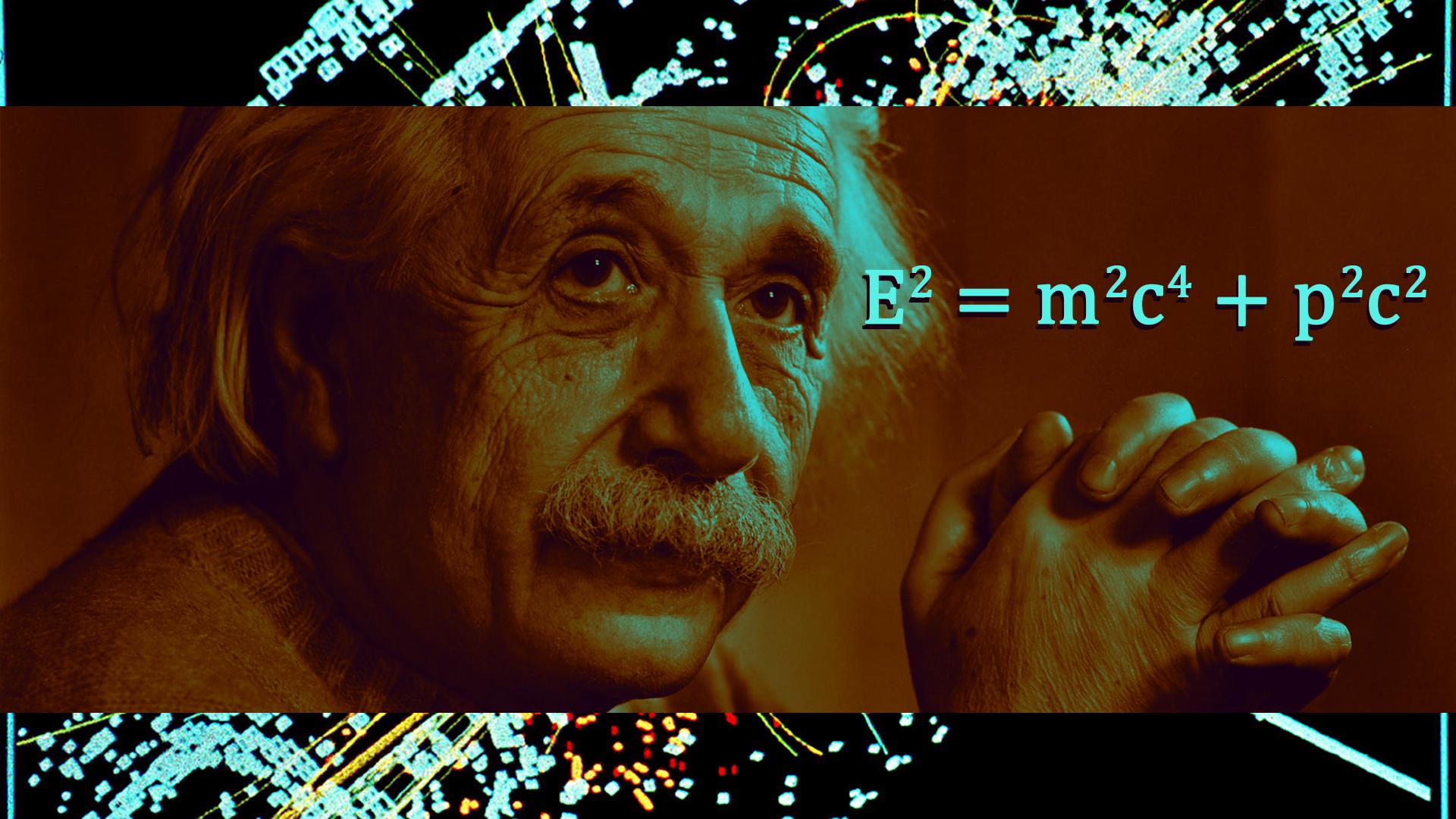
1906 - Rutherford did more experiments and in 1911 went before the Royal Society to explain the experiments propound the atomic nucleus.
1920 - Arthur Eddington proposes the mechanism of nuclear fusion process in stars.

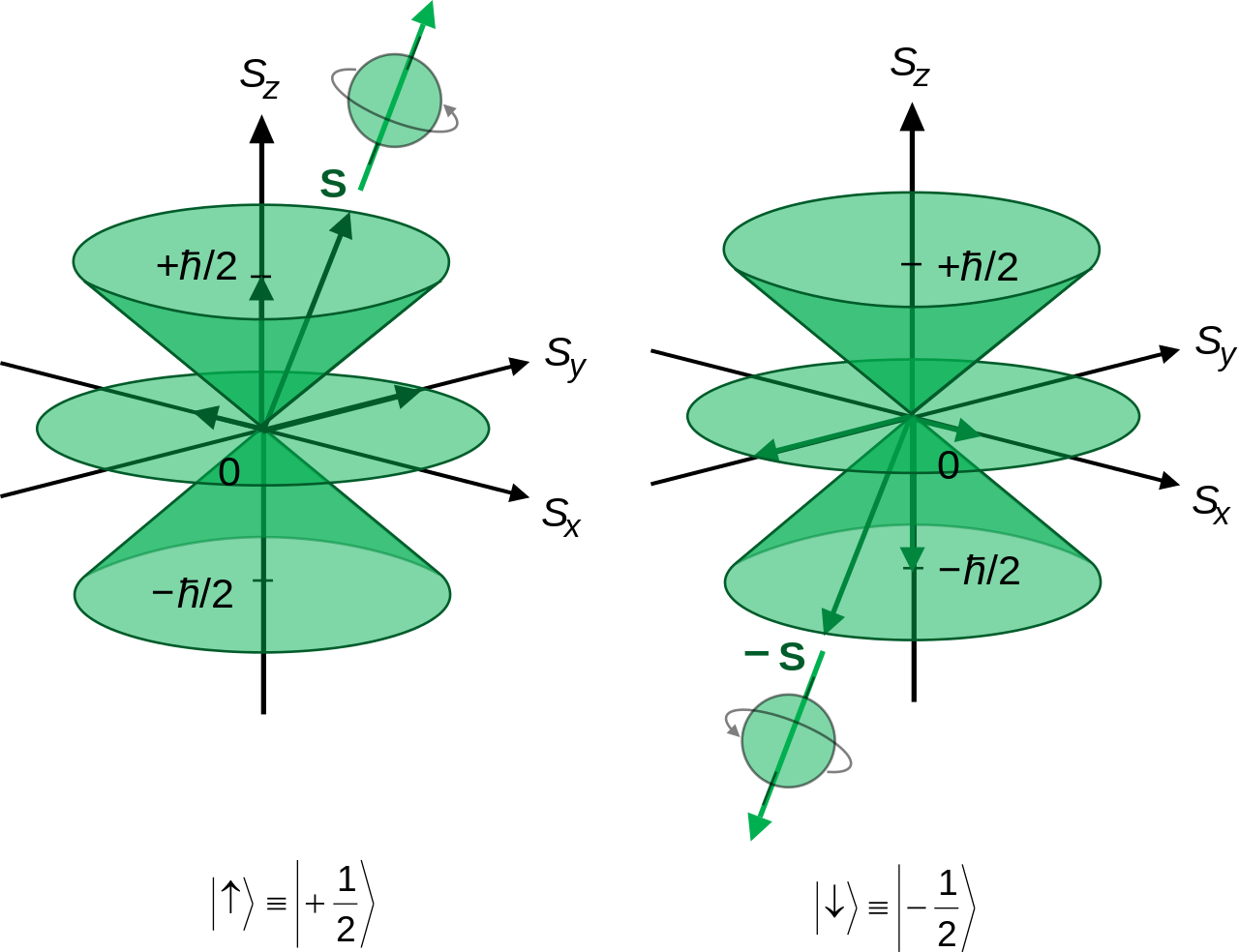
1925 - By this time, it was known that protons and electrons each had spin 1/2. Franco Rasetti perfomed studies of nuclear spin, discored that nitrogen-14 had spin 1.
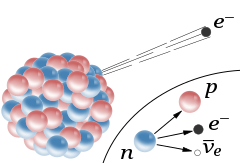
1932 - Chadwick discovers the neutron. Dimitri Ivanenko suggests that there were no electrons in the nucleus/neutrons were spin 1/2. This solve the nitrogen-14 spin problem.
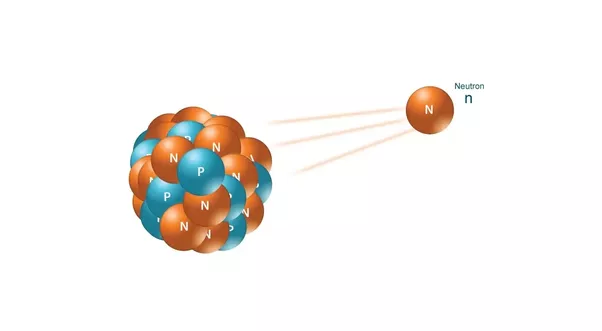
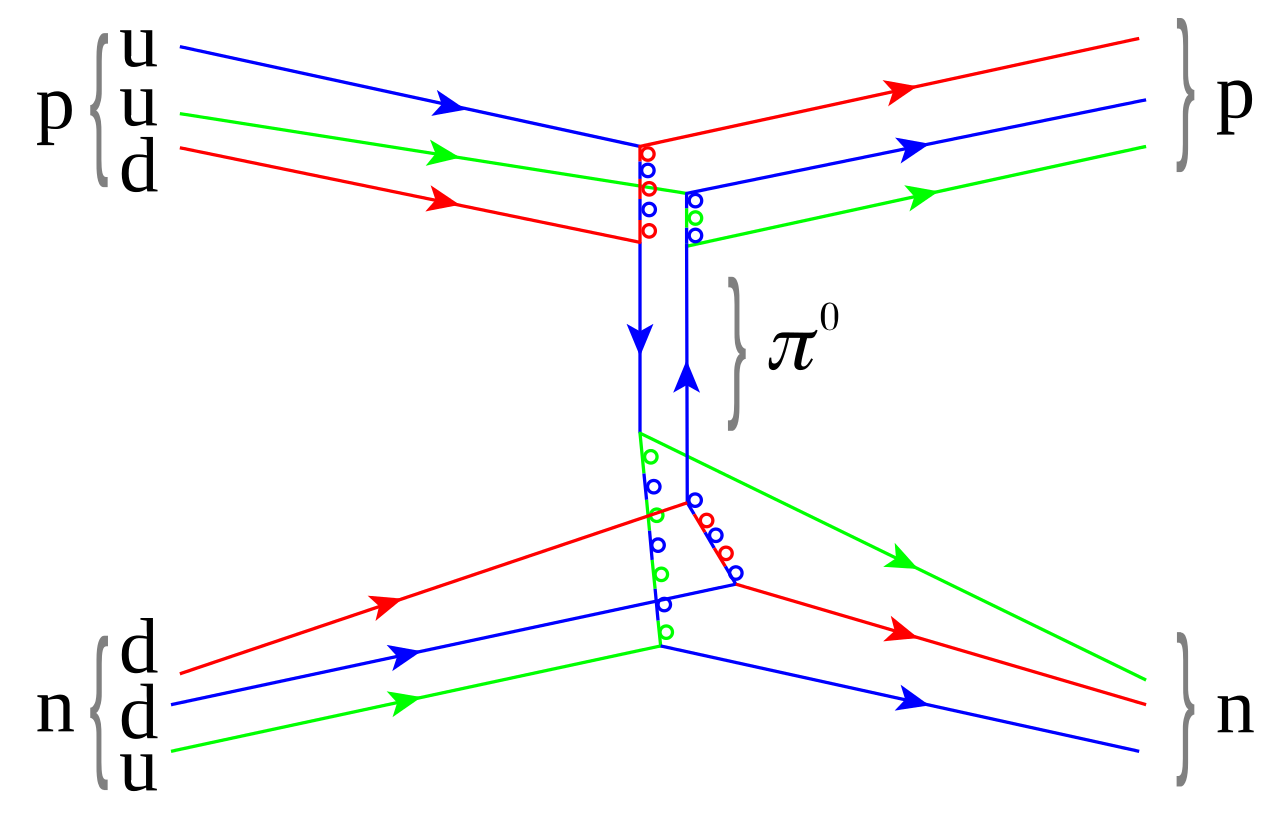
1935 - Hideki Yukawa proposes the theory of strong force - how the nucleus holds together.
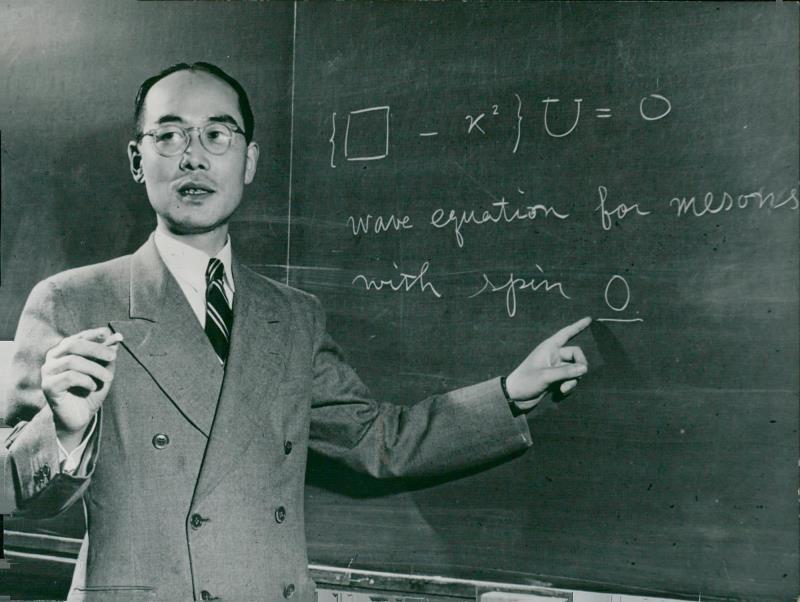
In the Yukawa interaction a virtual particle, meson, mediates the force between all the nucleons.
\[ V \approx g \bar{\psi} \phi \psi \qquad \rightarrow \qquad V(r)=-\frac{g^{2}}{4 \pi} \frac{1}{r} e^{-\mu r} \]
The discovery of the pi meson, showed the properties of the interaction.
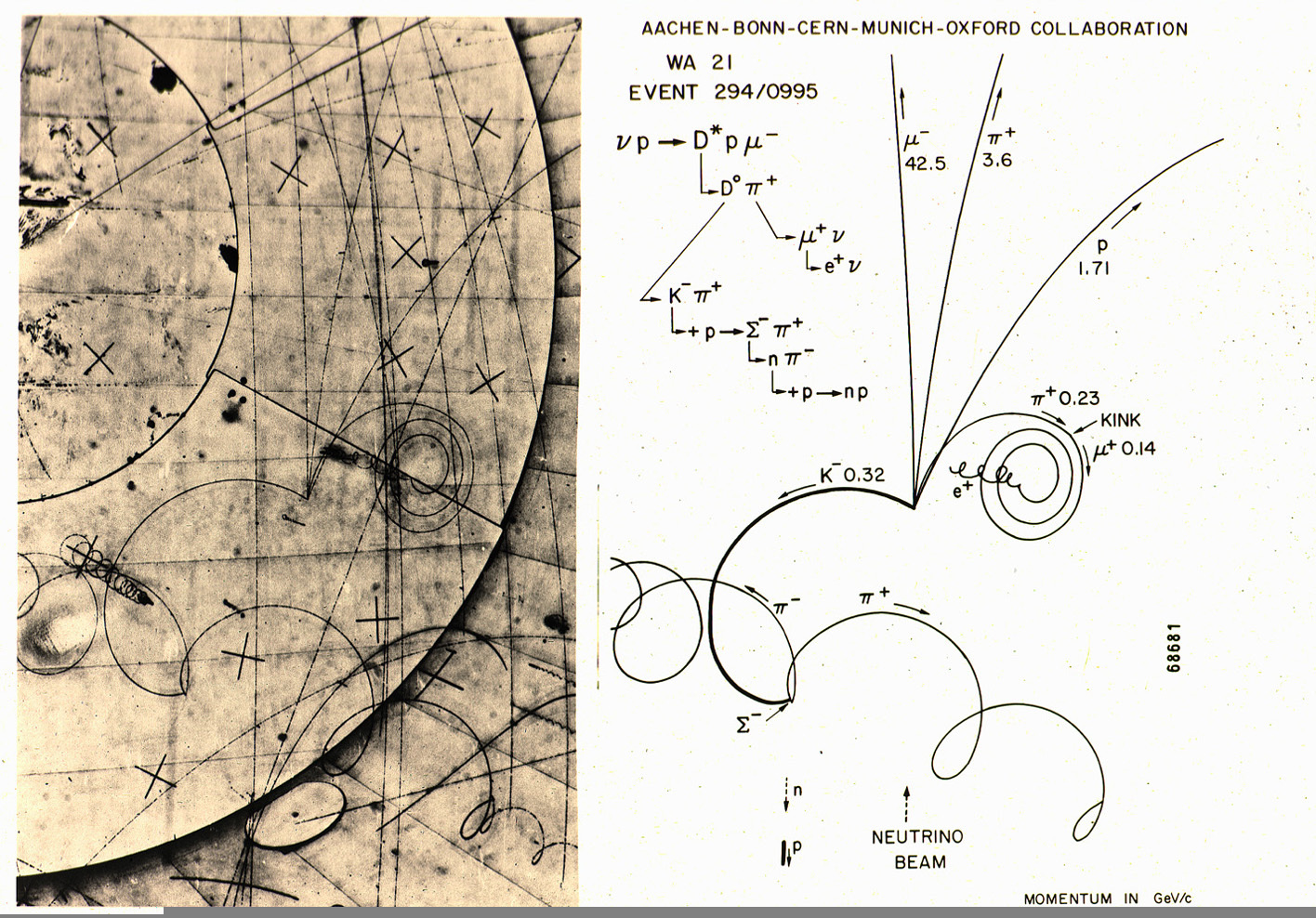
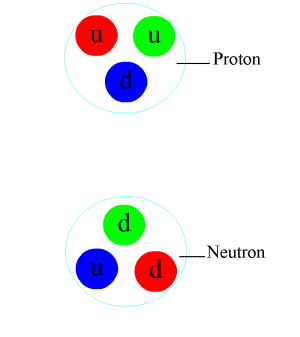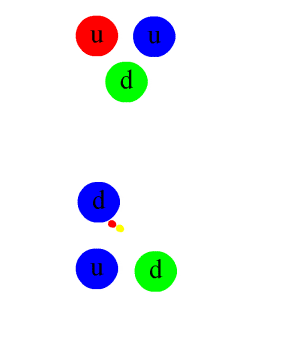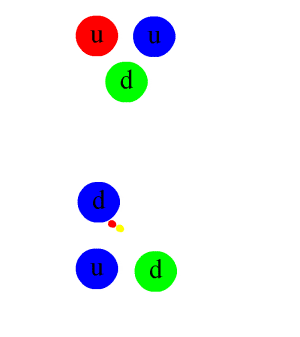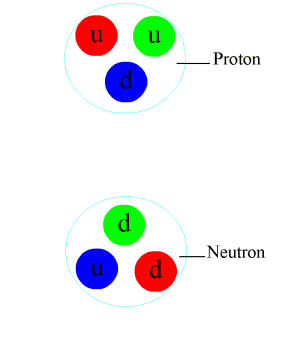
The modern model of the atom was complet. The atom's center contains a tight `ball` of neutrons and protons, held together by the strong force.
Terminology
A given atom is specified by the number of
- Neutrons: \(N\)
- Proton: \(Z\)
- Electrons: There are \(Z\) electron in neutral atoms
Atoms of the same element have same atomic number \(Z\). But they can be different. Isotopes of the same element have different # of neutrons \(N\).
Conventionally it can be represented as
\[^A_ZX\]
The mass number \(A\) is the total number of nucleons, i.e., \(A=N+Z\). For instance, \(^4\)He is the helium-4 nucleus, \(N=Z=2\), also called \(\alpha\).
Representation of a atom.
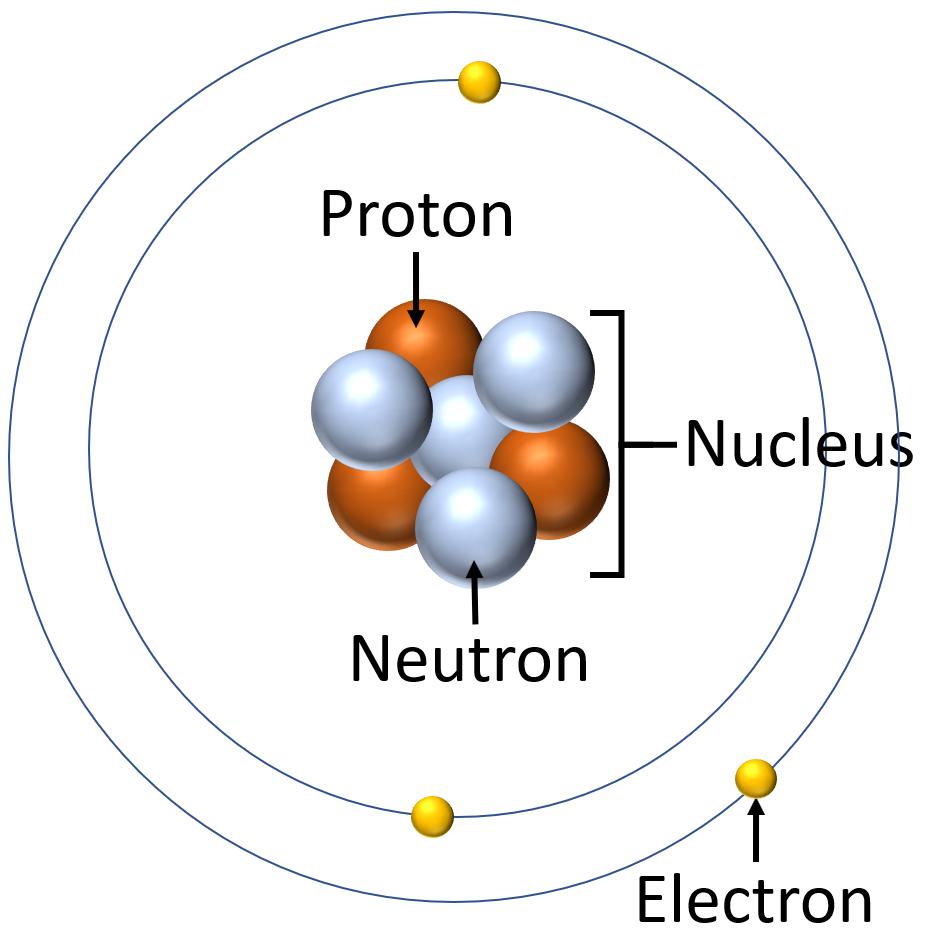
The nucleus diameter is \(\sim 10^{-15}\) while the atom is \(\sim 10^{-10}\) m, i.e., 1/100,000 of the size of the whole atom. A good comparisson would be a baseball ball in the middle of a stadium.
The nucleus contains more than 99.9% of the mass of the atom.
Modern Nuclear physics
Liquid-drop model
Heavy nucleus contain hundreds of nucleons. Sometimes is treated as a classical system.

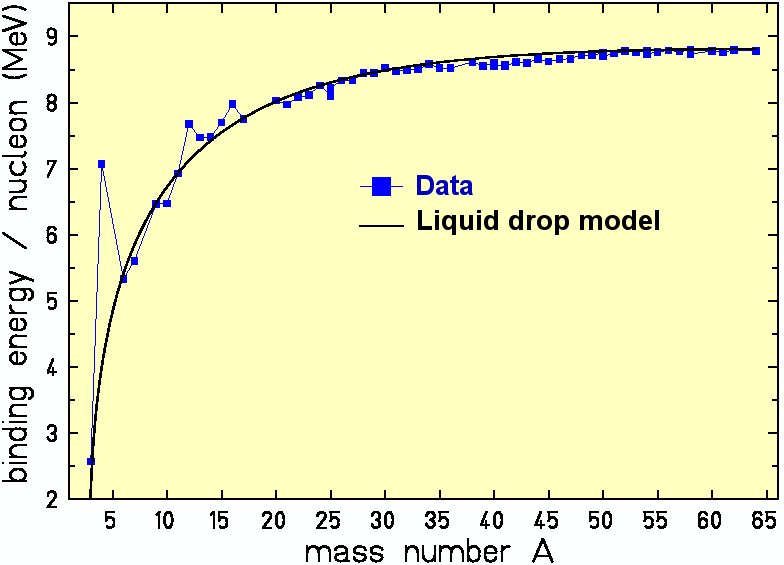
\(E_{\rm {B}}=a_{\rm {V}}A-a_{\rm {S}}A^{2/3}-a_{\rm {C}}{\frac {Z(Z-1)}{A^{1/3}}}-a_{\rm {A}}{\frac {(N-Z)^{2}}{A}}+\delta (N,Z)\)
\[ a_{\rm {V}} = 15.85; a_{\rm {S}} = 18.34; a_{\rm {C}} = 0.71; a_{\rm {A}} = 23.21\, {\rm MeV} \]
Nuclear shell mode
Considering quantum-mechanical effets → nuclear shell mode, developed in large part by Maria Mayer and J. Jensen (Nobel Prize 1963).
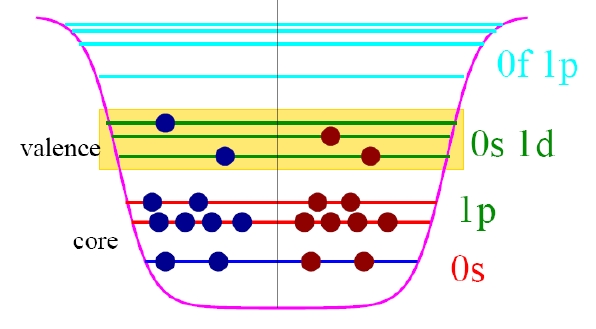
Magic numbers: \(\sum _{n=0}^{k}(n+1)(n+2)={\frac {(k+1)(k+2)(k+3)}{3}}\)
This gives the following magic numbers: 2, 8, 20, 40, 70, 112, ..., which agree with experiment only in the first three entries.
Interacting boson model
- More complicated models also exist, such as the interacting boson model.
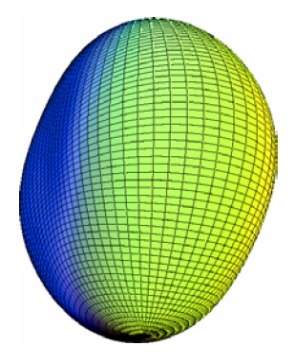
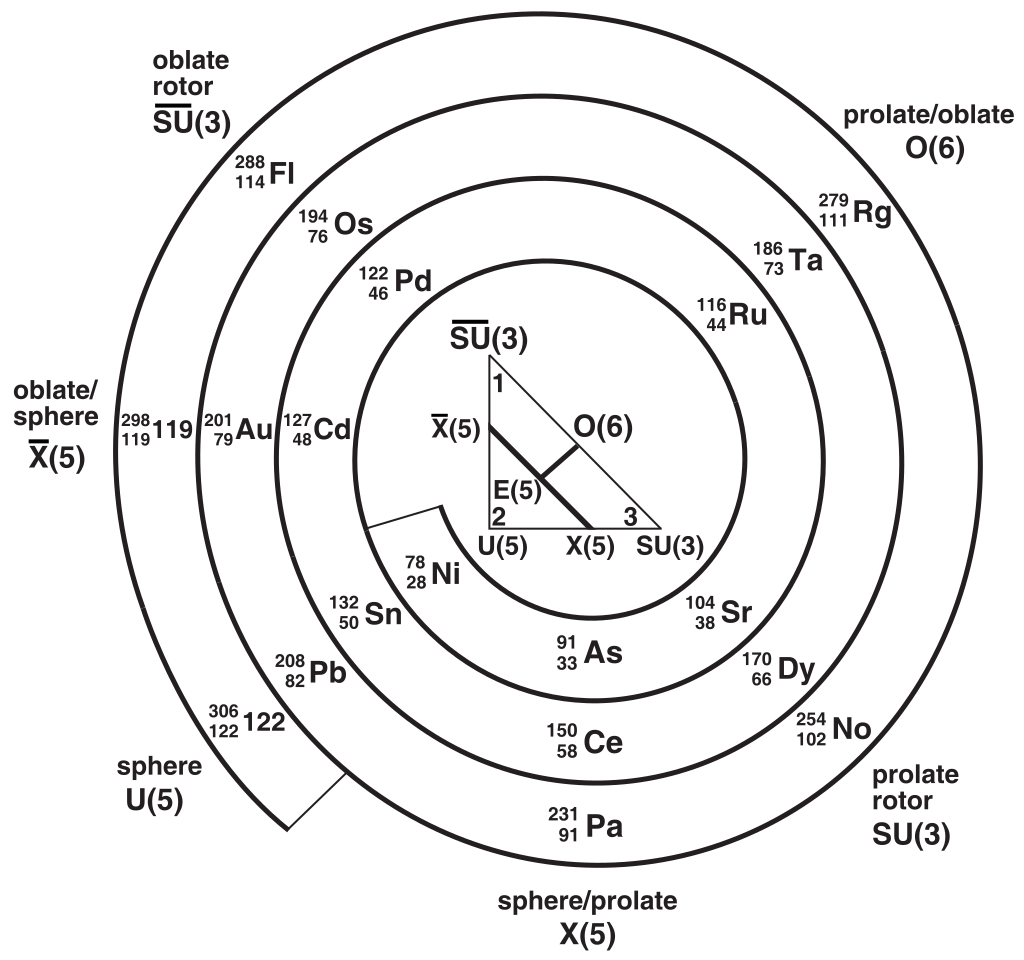
The model can be used to predict vibrational and rotational modes of non-spherical nuclei.
Quantum chromodynamics
- Ab initio methods try to solve the nuclear many-body problem from the ground.
\(\mathcal{L}_{\mathrm {QCD} }={\bar {\psi }}_{i}\left(i(\gamma ^{\mu }D_{\mu })_{ij}-m\,\delta _{ij}\right)\psi _{j}-{\frac {1}{4}}G_{\mu \nu }^{a}G_{a}^{\mu \nu };\quad G_{\mu \nu }^{a}=\partial _{\mu }{\mathcal {A}}_{\nu }^{a}-\partial _{\nu }{\mathcal {A}}_{\mu }^{a}+gf^{abc}{\mathcal {A}}_{\mu }^{b}{\mathcal {A}}_{\nu }^{c}\)
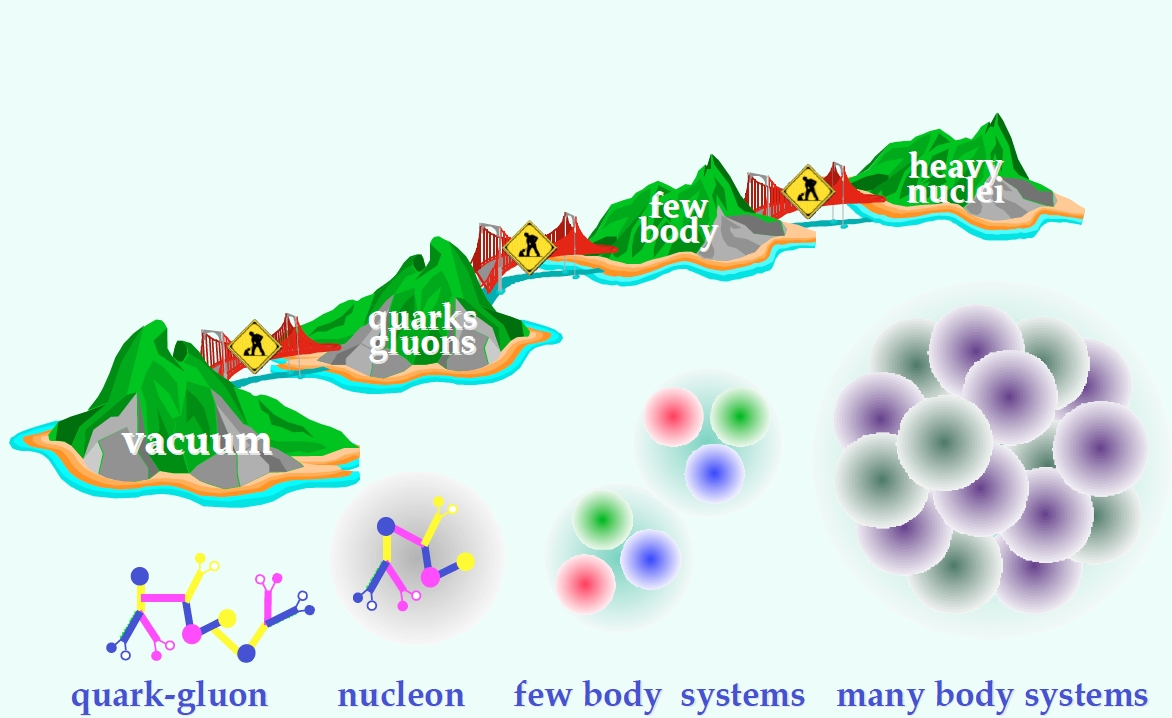
- From QCD to nuclei.
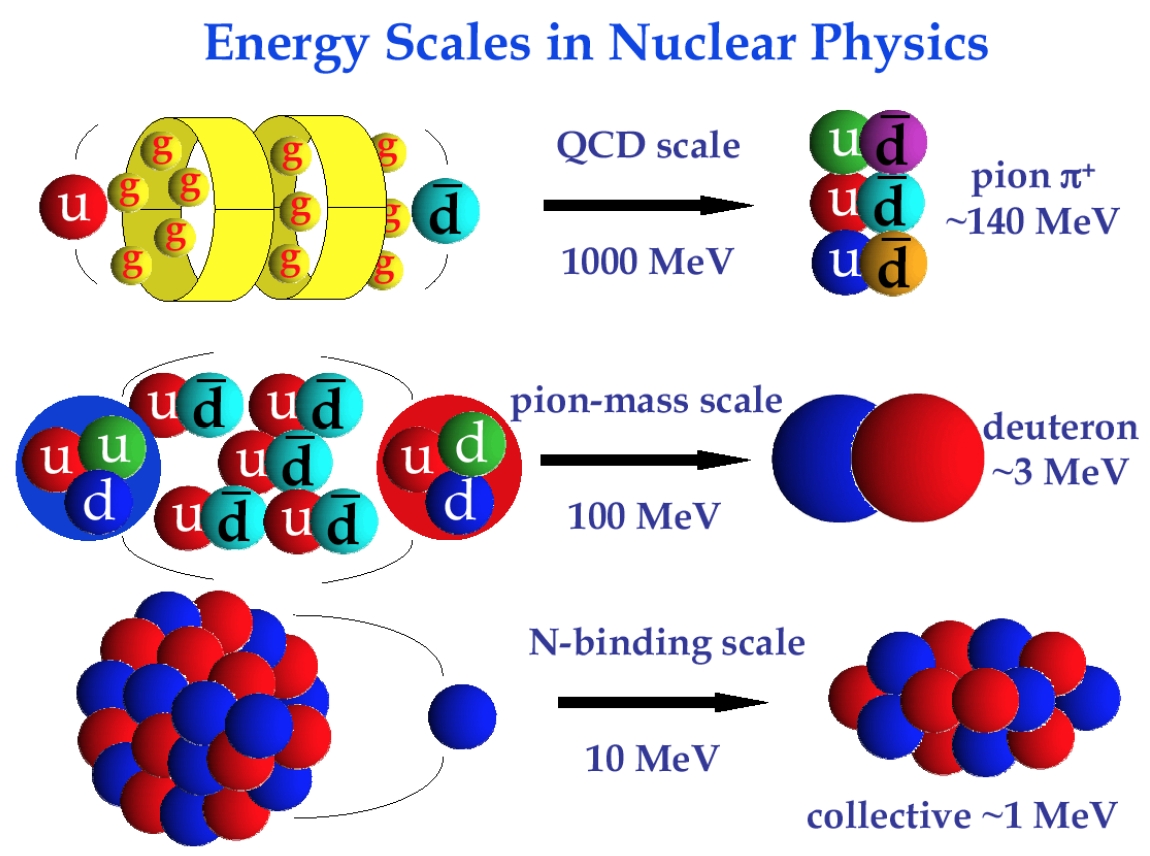
\({\rm \scriptscriptstyle{C.A. Bertulani\ and\ J. Dobaczewski}}\)
Fundamental interactions
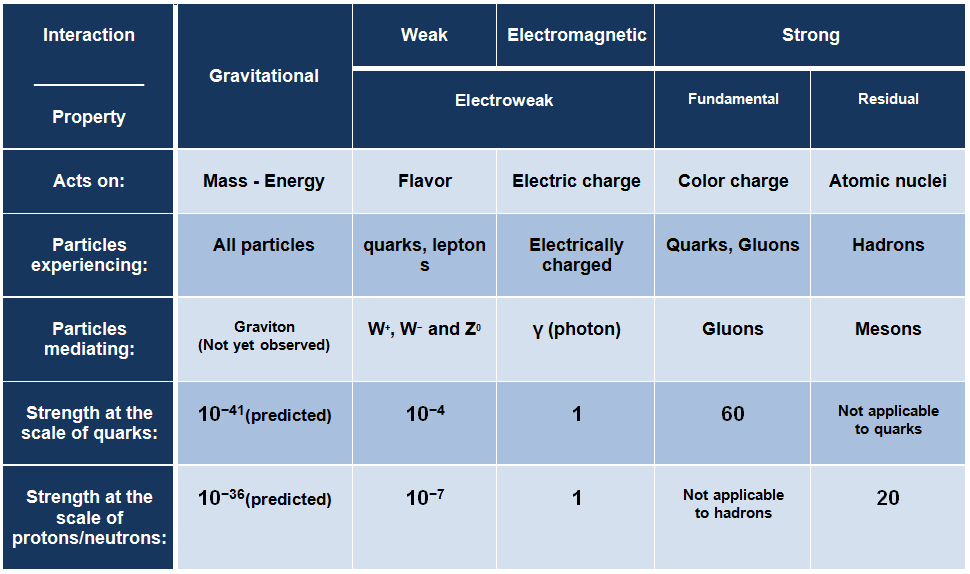
- Nuclear force.
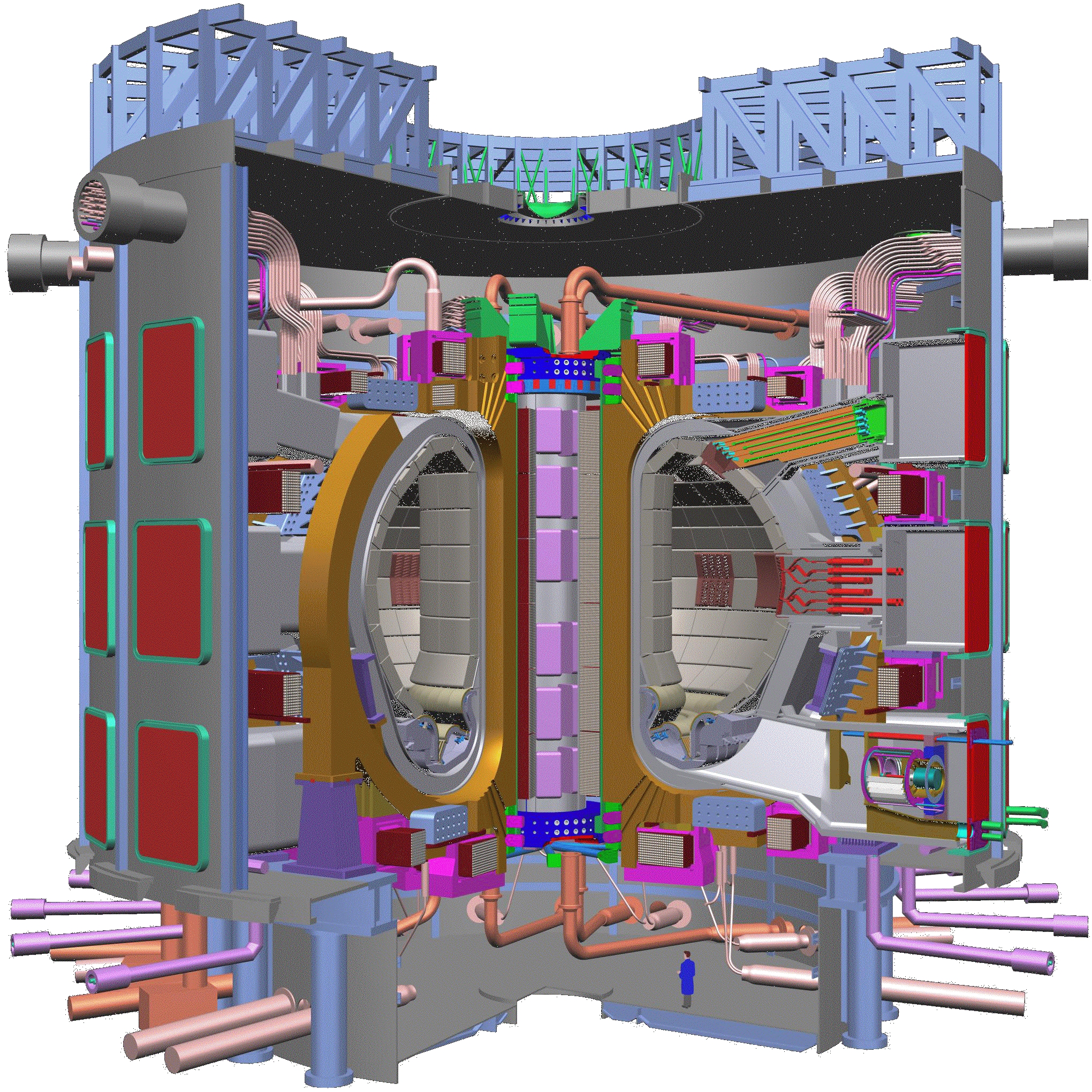
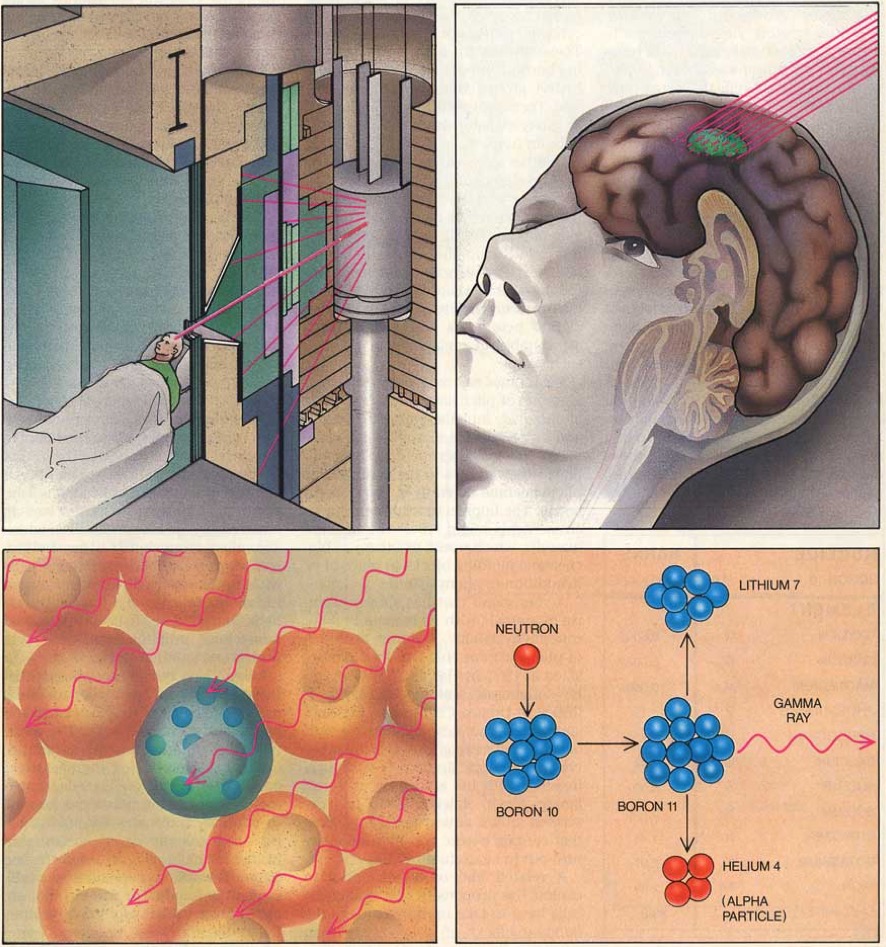
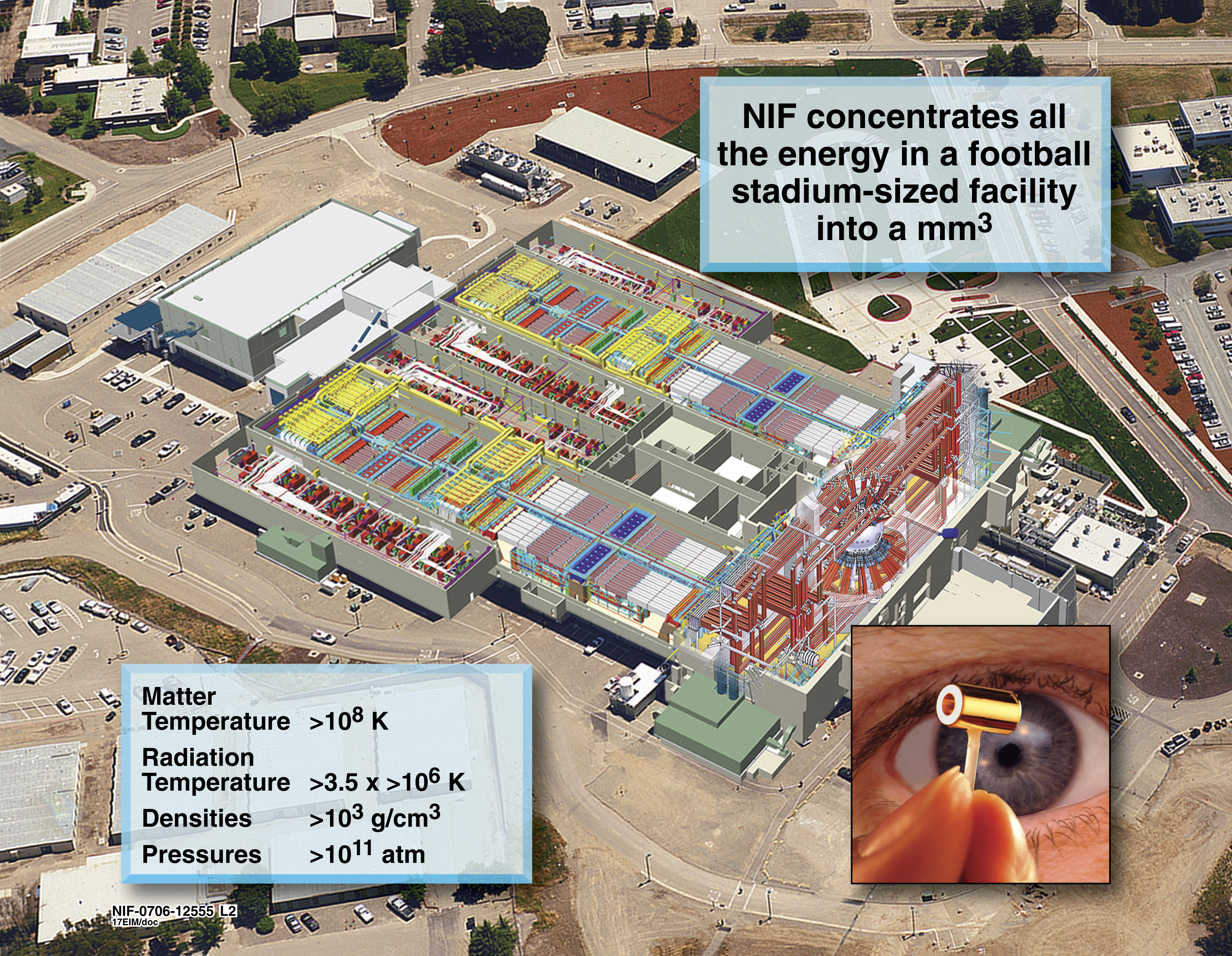
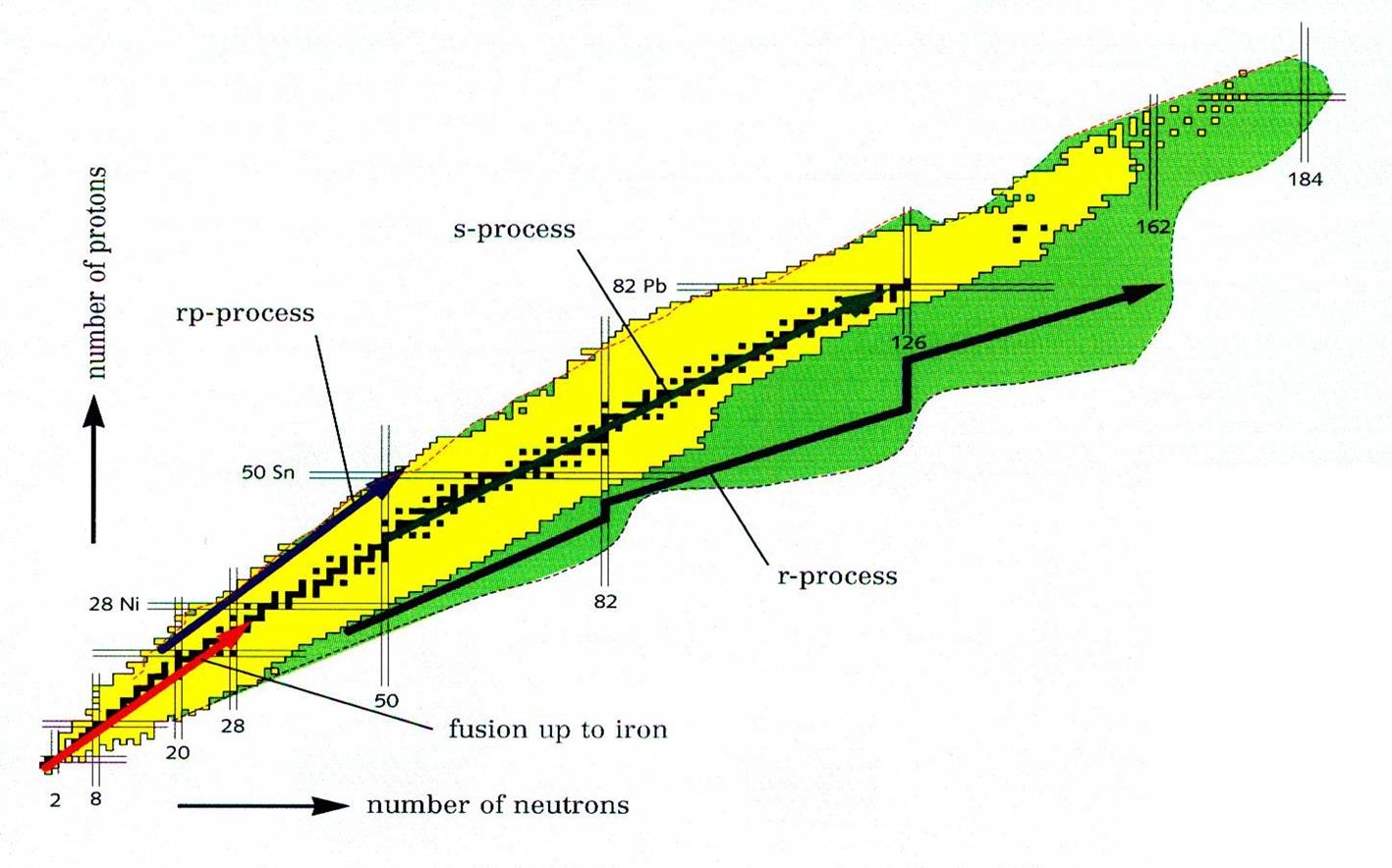
Applications of Nuclear Physics